Peter Greven: Eye on Lubricants

Lubricants and glidants play an essential role in the successful manufacturing of solid oral dosage forms. The process involves several unit operations such as blending, powder filling (capsules, sachets, stick-packs etc.), compaction and compression. A consistent and uniform flow of powder material through processing equipment into the final dosage form is critical during these operations to ensure a quality product. Typical formulation matrix is made up of a mixture of components that may differ in terms of their physical and morphological characteristics. Differences such as moisture content, particle size and shape, bulk density may cause particle adhesion and friction that can significantly reduce the powder flowability. Poor flow can cause insufficient mixing of the blends, (content uniformity issues) and rat-holing in the hopper of a tablet press (segregation issue), impacting both product quality and operation. Sticking to processing equipment is another common problem encountered by manufacturers. Lubricant in solid dosage forms is an additive used to reduce the friction between powder particles or between powder and the equipment surfaces.
Lubricants work by different mechanisms. However, boundary lubricants are more common while formulating solids. They typically work by forming layer or film between surfaces or at the interface to reduce the friction.
Magnesium stearate is the most commonly used solid lubricant in the pharmaceutical and nutraceutical solid oral dosages. It possesses an excellent lubricant capacity at very low use levels and is therefore generally referred to as gold standard boundary lubricant in the pharmaceutical and supplement industry. However, to make the best use of this material, one needs to understand the way it works and its physicochemical characteristics that can have significant impact on its lubrication efficiency. Commercial magnesium stearate is available from various suppliers in the form of various grades that differ in physical properties depending on the source and methods of production. Common processing related issues such as soft tablets, capping, delayed tablet dissolution can be easily avoided by properly understanding the properties of this material and thereby using it in the right manner. This article briefly touches upon some of these aspects and suggestions for using magnesium stearate and other lubricants in solid dosage forms.
Mechanism of Action
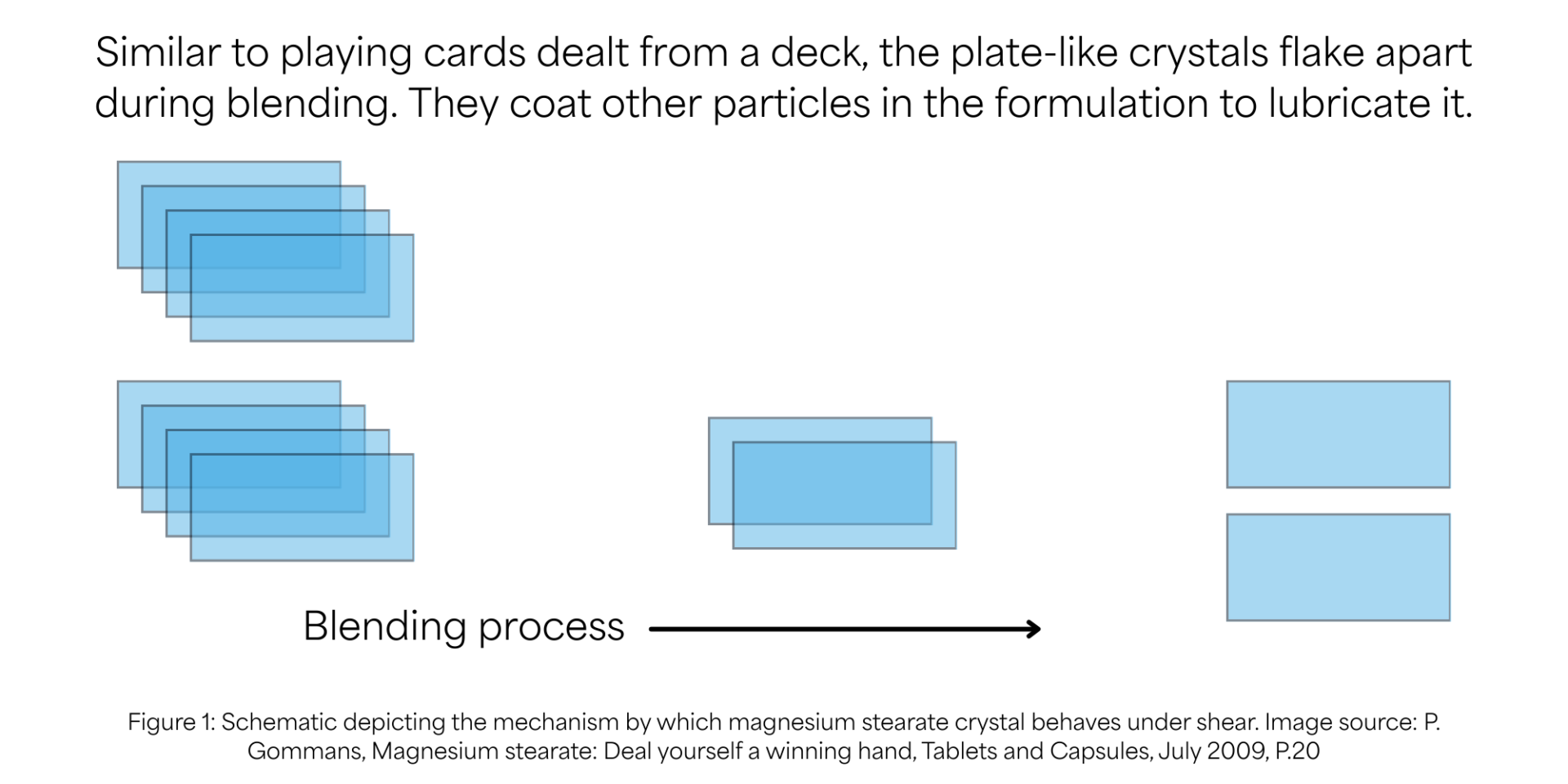
Magnesium stearate crystals are plate like structures arranged in a stack form like a deck of cards (figure 1). During blending these “plates” disengage and coat particles by forming a layer. Thus, the more plates disengage and coat other particles the higher is the lubrication efficiency. Interestingly, with magnesium stearate, higher lubrication does not always lead to better outcomes for the final dosage forms. In fact, over lubrication is the most common cause of the issues like softer tablets and delayed dissolution. Hydrophobic nature of magnesium stearate results in the formation of coating that tend to reduce particle adhesion during blending and compression resulting in softer tablets. It can also prevent water access during disintegration resulting in delayed dissolution. Therefore, identifying the right concentration and optimum lubrication time during initial process development is important to avoid issues post-production. Process development should also take into account physical properties such as particle size distribution, specific surface area, bulk density and lot to lot variation as these can significantly affect the extent of particle coating and therefore lubrication efficiency.
Manufacturing Process and Chemical Incompatibilities
Magnesium stearate is prepared from stearic acid that may be derived from animal or vegetable sources. Commercial stearic acid, which is typically a mixture of stearic, palmitic and myristic acid when treated with either magnesium oxide, carbonate or hydroxide yields magnesium stearate. Since the outbreak of bovine spongiform encephalopathy (mad cow disease), animal-source material is largely being replaced by vegetable-source material. Depending on the starting material used, related substances present in them and the manufacturing process, magnesium stearate can react with certain APIs like aspirin, ibuprofen, certain compounds containing primary amino groups, some vitamins and alkaloids. Thus, selecting the right source that uses the right starting material and process that can deliver the product with low impurity and high quality in a consistent manner is very important.
Physical and Chemical Properties
Magnesium stearate exists in several physical forms. Anhydrous amorphous form can absorb water and turn into crystalline form. Crystalline form can be mono-, di-, and trihydrate with dihydrate being the most efficient in terms of lubrication efficiency. Commercial magnesium stearate can be a mixture of different forms depending on the starting material, manufacturing process, storage conditions etc.
In addition to morphology, particle size distribution, specific surface area, bulk density also play an important role in determining the lubricant efficiency of magnesium stearate. Generally smaller particles have lower bulk density, higher specific surface area and higher lubrication efficiency. Thus, lot to lot consistency in raw material specifications is important. Lubrication efficiency of magnesium stearate is also highly governed by the mixing process and duration, the amount of shear, concentration used, and the stage of addition. Therefore, it is important to clearly define the blending process in manufacturing instructions to avoid errors during execution.
Manufacturing Process: Direct process vs. Controlled Precipitation
Commercially magnesium stearate is available in various grades and particle sizes. Particle size distribution and specific surface area play important roles in dictating the lubrication capacity of this material. Direct process involves mechanical size reduction methods such as milling to achieve the required particle size; however, material obtained by this process tend to have low specific surface area and high bulk density. On the other hand, controlled precipitation under right environmental condition following synthesis allows for better control over particle morphology and therefore it is possible to achieve material with high specific surface area and low bulk density which are both essential properties for a boundary lubricant.
In summary, following factors should be taken into consideration while selecting magnesium stearate as a lubricant.
- Physical properties: Good indicators are specific surface area, crystal structure. The higher the specific surface area, the more particles can flake off from planar metallic stearate crystals. Dihydrate form is the most efficient for lubrication.
- Blender type and blending time: Know the impact on the lubrication. The more shear force, the more particles can flake off the crystal. Also longer blending time result in the same effect but avoid over blending to minimize downstream processing issues.
- Concentration: Optimum concentration of a lubricant should be determined during the process development phase.
- Know the incompatibilities: Alternative lubricants such as stearic acid, other metallic stearates (e.g. calcium stearate, zinc stearate), fatty acid esters (e.g. glyceryl dibehenate, sodium stearyl fumarate) are commonly used when magnesium stearate can not be used.
- Selection of right supplier: Since lubrication efficiency of magnesium stearate is dependent on multiple factors as discussed, obtaining this material from a reliable supplier that ensure lot to lot consistency with respect to quality and material properties is crucial for successful manufacturing and avoiding unwanted issues during commercial production.
Peter Greven is the leading manufacturer and a global supplier of magnesium stearate and other solid lubricants. With their conscious sustainability driven efforts, Peter Greven supply vegetable grade metallic stearates that are produced by using sustainably grown palm oil and RSPO certified raw materials. Their LIGAMED® product line offers outstanding quality and purity lubricants that comply with all major pharmacopeias and are produced in accordance with IPEC PQG GMP conditions. This product line is manufactured with special precipitated production process that enables the high specific surface area and low bulk density. Peter Greven also offers lubricants manufactured via direct process under the brand name PALMSTAR®. In addition to these, they supply metallic stearates for applications in food and agriculture industries under the brands LIFAFOOD® and LIGAFEED® respectively.
To learn more about these products, visit our portfolio.
Discover the difference Peter Greven can make in your formulations.
References
R. Dansereau and G. Peck, The effect of the variability in the physical and chemical properties of magnesium stearate on the properties of compressed tablets, Drug Dev Ind Pharm, 1987(13) 6, 975-999.
K. Ertel and J. Carstensen, Chemical, physical and lubricant properties of magnesium stearate, J Pharm Sci, 1988 ( 77) 625-629.
V. Swaminathan and D. Kildsig, Examination of the moisture sorption characteristics of commercial magnesium stearate, AAPS PharmSciTech, 2001 (2) 4, article 28.
P. Gommans, Magnesium stearate: Deal yourself a winning hand, Tablets and Capsules, July 2009, P.20




