The Nitrosamine Challenge: Why Excipient Choice Matters More Than Ever

What are Nitrosamines?
Nitrosamines are a class of chemical compounds that can form unintentionally during the manufacturing of pharmaceuticals. Some nitrosamines are classified as probable human carcinogens by regulatory agencies, such as the FDA and EMA. Their presence in drug products has led to global recalls of medicinal products and heightened scrutiny of raw materials, including excipients. Regulatory authorities have identified unacceptable levels of nitrosamine impurities in various active pharmaceutical ingredients (APIs) and other approved medications leading to the abrupt recall of drugs like sartans (valsartan), ranitidine (zantac), nizatidine, metformin, and varenicline [Bharate SS 2021].
Presence of vulnerable amines, such as secondary and tertiary amines are known to accelerate the formation of nitrosamines under certain conditions [Ashworth IW 2020]. These amines react with nitrous acid in acidic conditions, leading to the formation of nitrosamines. Nitrous acid itself is unstable and is formed from nitrites (NO2) under acidic conditions. Other sources of nitrosamine impurities include vendor-sourced raw materials, intermediates, and the reuse of solvents, reactants, catalysts and reagents during synthesis. Therefore, during the manufacturing of drug products, several factors, such as the selection of additives, preservatives, and excipients, should be carefully assessed. Further, storage conditions post manufacturing such as temperature, moisture, product pH might also provide conducive environment to generate nitrosamine impurities. Formulators should consider all these factors while developing new products.
Nitrosamine impurities are classified as Class 1 known mutagenic impurities with potential to be carcinogenic according to (ICH) M7 guidelines. Several regulatory authorities issued guidance (EMA, FDA) and information on Nitrosamine impurities and requested Marketing Authorization Holders (MAHs) to conduct a risk evaluation with regards to Nitrosamine formation in their drug products.
Risk evaluation for nitrosamine formation in drug product involve a comprehensive assessment of all steps and material involved during and after the drug development process. This begins with identification of potential sources of nitrosamine formation which include raw materials and excipients, manufacturing process, storage conditions and equipment/facility design. Source identification is followed by implementing appropriate control strategies that include process optimization, selection of low-risk excipients, testing and monitoring with appropriate analytical methods.
Excipients are an essential component of most medicinal products and therefore, selection of low nitrosamine risk excipient is critical part of risk assessment.
A “Low Nitrosamine Risk” Excipient:
- Has amine and nitrite levels consistently below detectable limits (sub-ppm range).
- Is manufactured under controlled conditions to limit contamination.
- Is tested and qualified using validated analytical methods.
- Has low residual solvents that could contribute to nitrosation reaction.
Excipient nitrite limits are not explicitly defined by a single universal standard but rather are assessed based on risk assessment as described above and established guidelines for nitrosamine formation in drug products. Manufacturers assess nitrite levels in excipients as part of a broader strategy to mitigate the risk of nitrosamine impurities. Excipient manufacturers are not required but are rather encouraged to test their excipients for nitrite content and share this data.
Lactose is one of the major excipients that forms an essential part of various medicinal, supplements and food products. Derived from whey, which is of natural origin, lactose in general is expected to have low nitrite levels. MEGGLE is the leading manufacturer of pharma and food grade lactose that is commercially available in various forms and grades. Historically known for its commitment towards producing high quality products, MEGGLE has not only launched low nitrite grades of lactose but has also invested heavily to develop a robust ion chromatography (IC) method to accurately quantify nitrite at trace levels, whereby the limit of quantification (LOQ) is 0.03 ppm.
In the publication from Boetzel et al. [2022], the nitrite content for Lactose from eight suppliers ranged from 0.07 to 1.7 ppm, with a mean of 0.54 ppm (table 1).
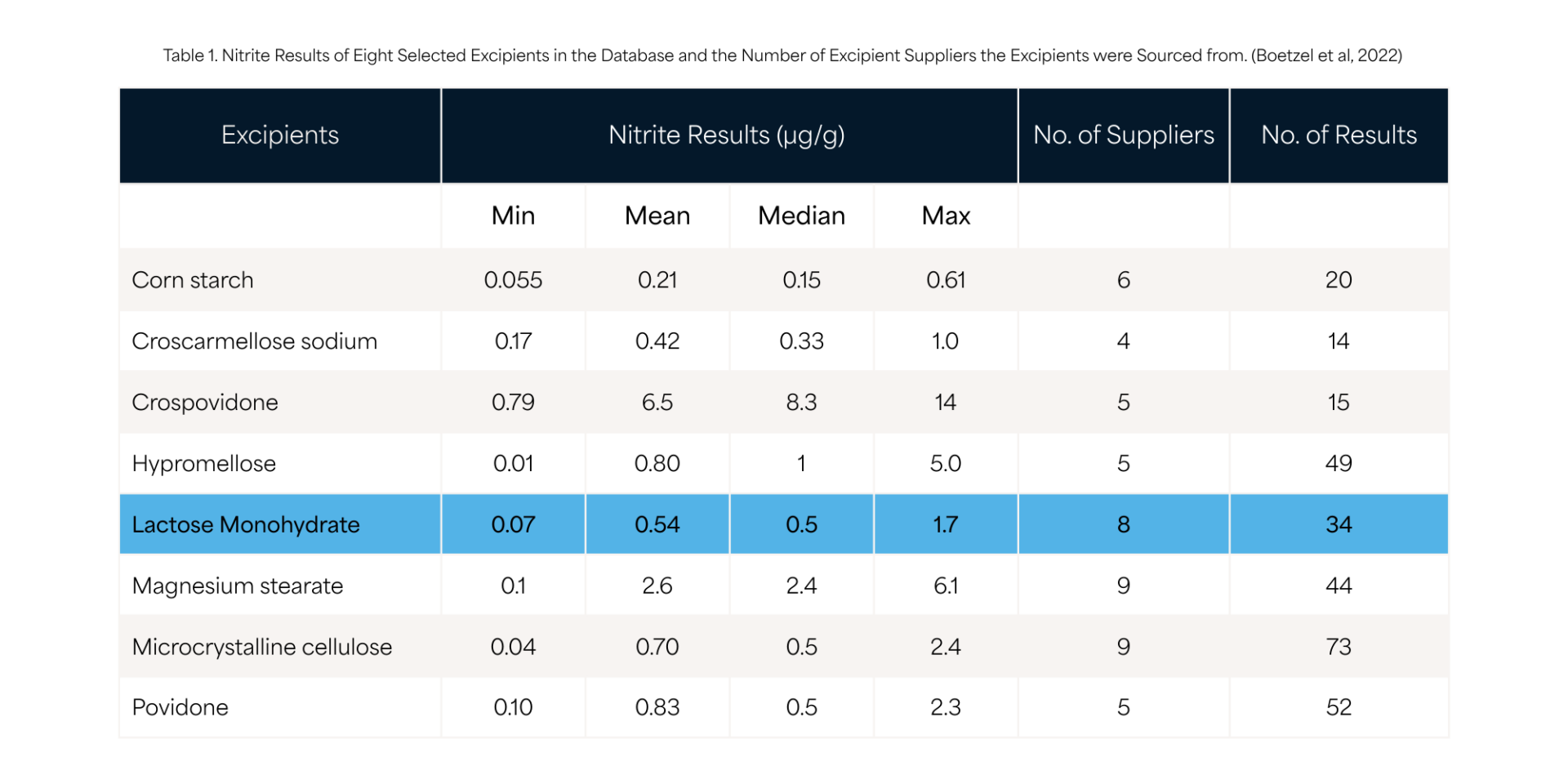
However, as the nitrite contribution is assumed as additive, the sum and total amount of all excipients in the formulation must be considered. As fillers/diluents are typically used in larger proportions, they could play an important role even if their nitrite content is comparably low.
MEGGLE’s IC method is much more sensitive than the recently published IC method for lactose in the application note from USP (Nitrite LOQ 0.2 ppm). Through additional validation, they have demonstrated that it can safely specify a limit of ≤ 0.10 ppm for low nitrite grades of lactose.
MEGGLE measures the nitrite parameter on each lot of the Low Nitrite grades. The nitrite limit of 0.10 ppm is part of the Product Specification and the measured value is part of the corresponding Certificate of Analysis (CoA). This not only simplifies the risk assessment for customers but also demonstrates compliance with the defined nitrite limits for critical products.
MEGGLE has launched the following premium products with a specified nitrite limit of ≤0.10 ppm for risk mitigation of nitrosamine formation in drug products:
- GranuLac® 200 Low Nitrite
- GranuLac 230® Low Nitrite
- Tablettose® 100 Low Nitrite
- FlowLac® 100 Low Nitrite
Ready to learn more?
References
Boetzel, R., Schlingemann, J., Hickert, S., Korn, C., Kocks, G., Luck, B., Blom, G., Harrison, M., François, M., Allain, L. R., Wu, Y. & Bousraf, Y. (2023). A Nitrite Excipient Database: A useful Tool to Support N-Nitrosamine Risk Assessments for Drug Products. Journal of Pharmaceutical Sciences. Vol 112 (6) P1615 – 1624.
Ricarda L., RISK MITIGATION OF NITROSAMINES FORMATION IN DRUG PRODUCTS:Role of Excipients. A White Paper by Meggle.
Hemanth P.R.V. et al. (2024). Nitrosamines crisis in pharmaceuticals Insights on toxicological implications, root causes and risk assessment: A systematic review. Journal of Pharmaceutical Analysis. Vol 14.
Bharate S.S. (2021). Critical analysis of drug product recalls due to nitrosamineimpurities, J. Med. Chem. Vol 64, P2923 – 2936.
Ashworth I.W., Dirat O., Teasdale A., et al. (2020), Potential for the formation of Nnitrosamines during the manufacture of active pharmaceutical ingredients: An assessment of the risk posed by trace nitrite in water, Org. Process Res. Dev. Vol 24 P1629 – 1646.




